
So, molecules are collections of atoms which are held together bythe attraction of one nucleus to the electrons of another atom. (This is the basis for the octet rule, arule which is violated very often by-the-way.) Ī C nucleus simply does not have enough positive charge toeffectively attract that eleventh electron, because (1) the electronis farther away, and (2) there are ten electrons shielding theeleventh electron from the nucleus that is, the eleventh electron isnot attracted because the cloud of negative charge between it and thepositively charged nucleus.
ATOMS JOIN TOGETHER TO FORM MOVIE
Step through through the QT movie by clicking the frame advance button. ( This is not really where the electrons go but it will demonstrate the point we want to make.)
ATOMS JOIN TOGETHER TO FORM FULL
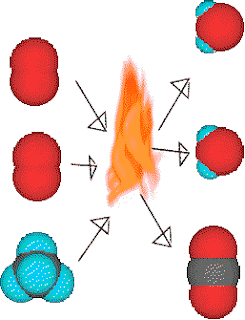
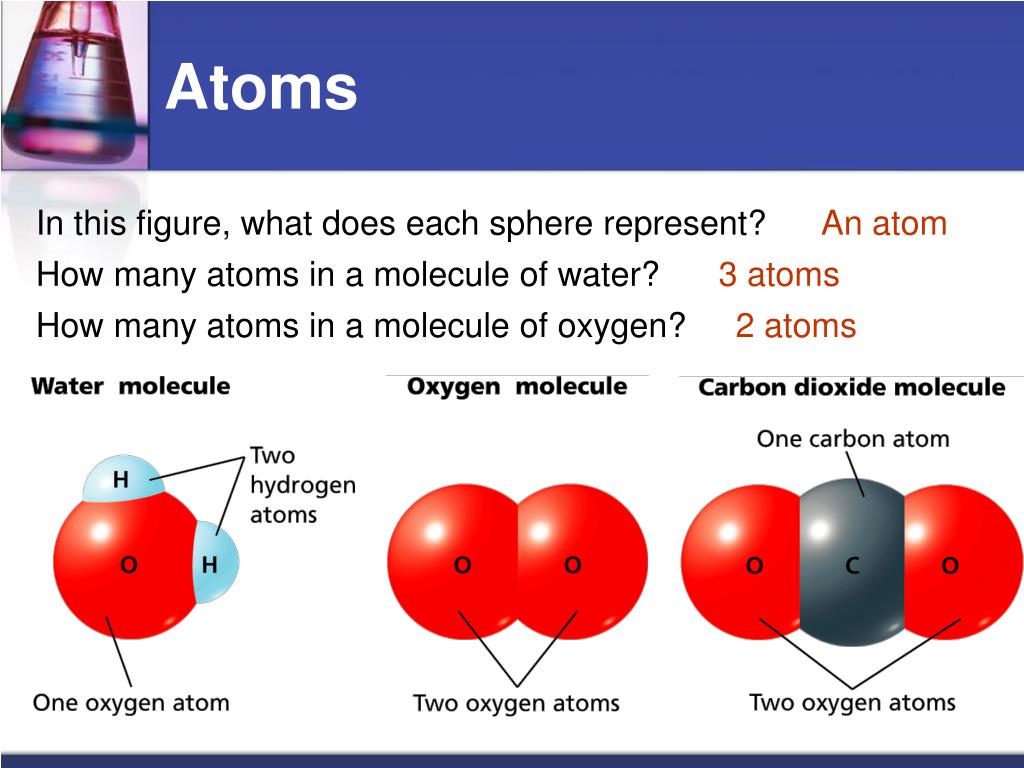
The n=2 shell is full the n=2 shell can hold 8 electrons, 2 in the s orbital and 6 in the p orbitals. The eleventh electron would have to go into the next principal quantum level, n=3. The next eight electrons fit into the n=2 shell.Īdding the fifth H to the molecule would increase the count of electrons around the C atom to eleven. A molecule is a group of atoms held together with chemical bonds. The first two electrons fit into the n=1 shell. Where are these ten electrons going to go? With four H's present there are ten electrons aroung the C atom. Lets stop and count electrons around the carbon and think about where they are. If a fifth H atom comes by, its electrons will not experience an attraction to the C nuclii. Ionic bonds form when two or more ions come together and are held together by. The same thing can be said for methane, CH 4.Ī C atom will attract electrons until four H's have taken position around the C atom. Covalent bonding involves the sharing of electrons between two or more atoms. So, the energy of two hydrogenatoms is lower when the two atoms are together than when the twoatoms are apart that is why they stay together. Single sided arrows represent attraction.īecause the electrons are attracted to both nuclii pulling the twoatoms apart would require energy. If the atoms get two closethen the nuclii will repell each other. If two atoms get close enough together then the electrons of eachatom will be attracted to both nuclii. When the electrons move, the current can flow through the system.Since electrons are negative they are attracted to the nucleusbecause it is positive. The electrons then jump from one area to another. The negatively charged pieces of any circuit have extra electrons, while the positively charged pieces want more electrons. The electrons move from negatively charged parts to positively charged ones. The very small particles can stream through wires and circuits, creating currents of electricity. Atomicity is defined as the number of atoms joined together to form a molecule of an element.
Both types of bonds have specific advantages and weaknesses.Įlectrons are very important in the world of electronics.
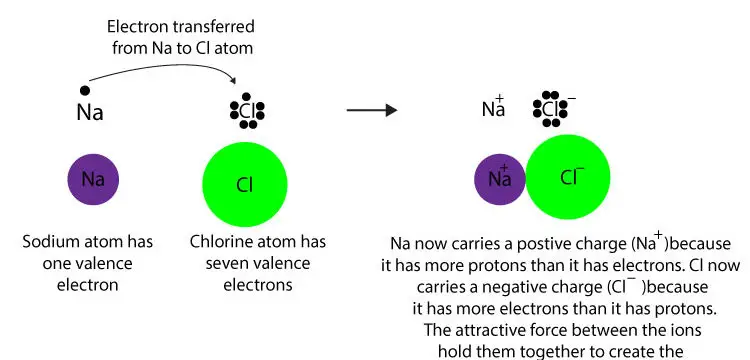
The second type of bonding is called covalent bonding, where electrons are actually shared between two or more atoms in a cloud. You wind up creating two ions as one atom loses an electron and one gains one. There is one type of bonding called electrovalent bonding (ionic), where an electron from one atom is transferred to another atom. The overall shell shape will also be more complex (because of the suborbitals) as you have more electrons.Įlectrons play a major role in all chemical bonds. The higher the atomic number, the more shells and electrons an atom will have.
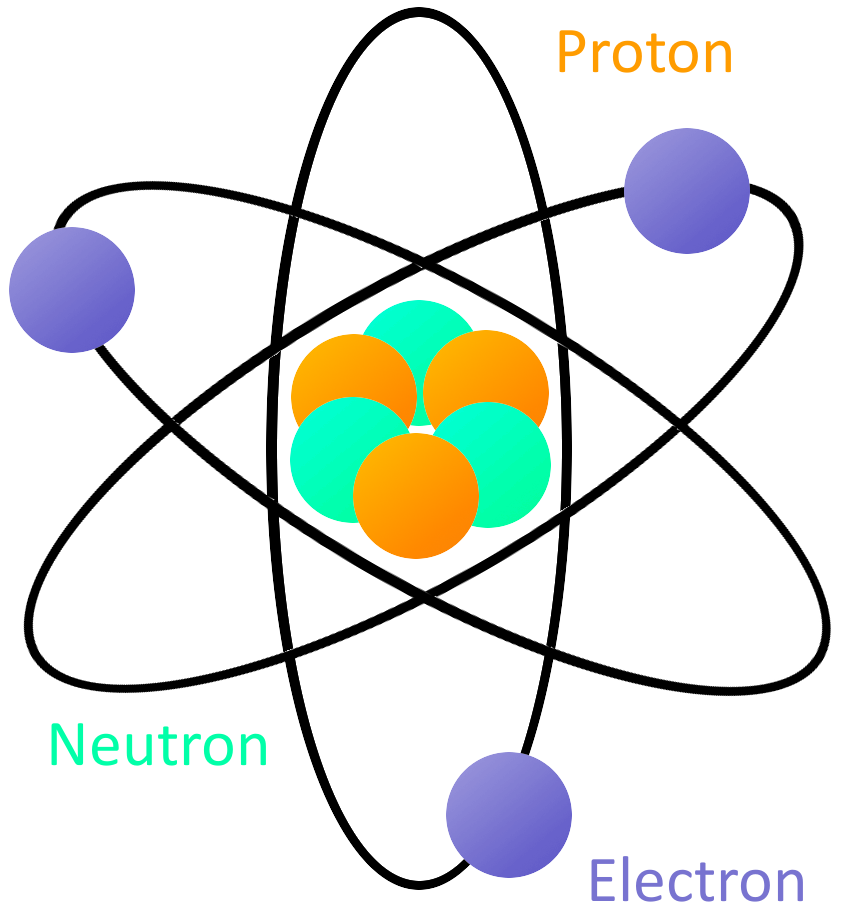
The overall shape of the shells changes depending on how many electrons an element has. After years of experimentation, scientists discovered specific areas where electrons are likely to be found. Because electrons move so quickly, it is impossible to see where they are at a specific moment in time. Those clouds are specific distances away from the nucleus and are generally organized into shells. The mass of an electron is almost 1,000 times smaller than the mass of a proton.Įlectrons are found in clouds that surround the nucleus of an atom. Electrons are extremely small compared to all of the other parts of the atom. Together, all of the electrons of an atom create a negative charge that balances the positive charge of the protons in the atomic nucleus. Electrons are the negatively charged particles of atom.


 0 kommentar(er)
0 kommentar(er)
